BrainsGate's protocol for treating VaD patients was approved by several Ethical Committees in India. It is an open-label, multi-center, rater-blinded pilot clinical study, which involves an elective implantation procedure of the INS. After 1-3 days of post implantation hospitalization, the patient is discharged to their home and undergoes four hours of daily stimulations during 3 months, at which stage the device is removed. Cognitive function, degree of cerebral blood perfusion and level of cerebral metabolism are evaluated at baseline and at several follow-up dates (up to 3 months post implantation). The study is planned to recruit up to 24 patients, and its endpoints are safety, tolerability and effectiveness of SPG stimulation for the treatment of Vascular Dementia.
|
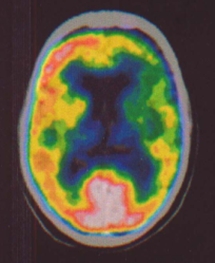
To date 11 patients were recruited into this study and most have already completed the treatment and follow-up phase. Initial results indicate a difference in brain perfusion between the treated and non-treated hemispheres. This can be seen in the following PET image, taken 12 hours after the last stimulation session. The treated hemisphere (on the left of the picture) can be seen to have more metabolic activity (brighter colors) as a result of the increased blood flow.
|  |  |
|
July 15, 2014
ImpACT24 Interim Results
The Data Safety Monitoring Board (the "DSMB") for BrainsGate's study examined the interim results and deterimined the results support the continuation of the study > full story
January 1, 1970
> full story
|

